What are Elements that exist as Gas, Liquid, Solid at Room Temperature?
States of Matter
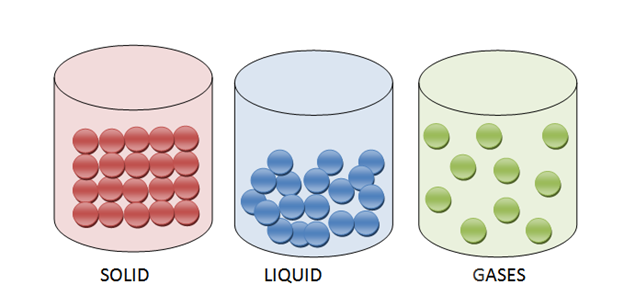
There are four types of states of matter. Solid, liquid, gas, and plasma. Some elements exist in solids, such as metals. Some are liquid such as h2o, mercury, and some a present in a gaseous state such as oxygen, hydrogen. There is another matter known as plasma, but we cannot find it on earth.
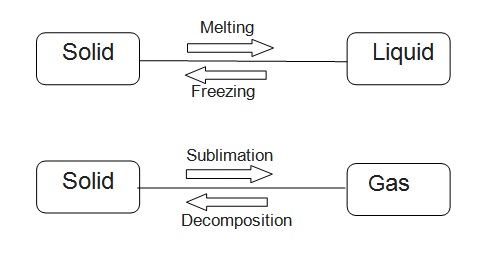
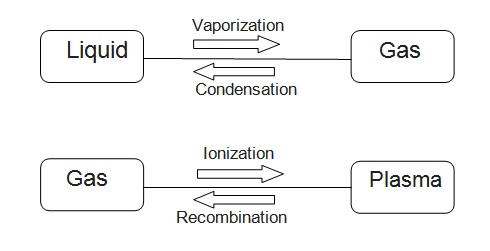
Inter-relation
Solids
Solid is a state of matter that has a fixed shape and volume. The molecules are closely packed.
Liquid
Liquid has loosely packed molecules. It acquires the shape of the vessel it is pouring in. Moreover, it has a fixed volume.
Gas
Gas is one of the four states of matter, including solid, liquid, and plasma. A gas can be made up of individual atoms, element molecules, and compound molecules.
Air is a mixture of different gases. Atoms of gases are loosely connected.
Difference between Solid, Liquid, and Gas
Shape
A solid has a fixed shape and cannot be easily changed. For example, a stone won’t change its shape easily.
On the other hand, liquid and as takes the shape of the containers.
Volume
Solid and liquid have a fixed volume. For example, stone or water will have the same volume no matter in which container it is.
While a gas has a variable volume, air can occupy a room, box, or even a small balloon.
Density
The densities of gases are lower than those of liquids and solids. Moreover, it changes with the temperature change.
Compressibility
The volume of solid or liquid will not change no matter how much pressure is applied. On the other hand, the volume of gases changes when high pressure is applied to it.
Intermolecular difference
- The molecules of solid are tightly or closely packed.
- The molecules of liquids are loosely bound to each other.
- The molecules of gases are super loose.
What are the Elements that exist as Liquids at Room Temperature?
Two elements are liquid at room temperature: Mercury and Bromine.
In a slightly higher temperature (25-40 degrees Celsius), six elements stay as liquid: Mercury, Bromine, Francium, Cesium, gallium, and Rubidium.
Mercury
At room temperature, exposed elemental mercury can evaporate to become an invisible, odorless toxic vapor. It is a shiny and silvery metallic liquid. It has a melting point of -38 degrees Celsius and a boiling point of 356 degrees Celsius.
Bromine
Bromine is a reddish-brown liquid halogen. Its melting point is -7.2 °C, and its boiling point is 58.8 °C.
Francium
Francium is liquid at a slightly higher temperature than room temperature. Its melting point is 27 °C ad boiling point is 677 °C.
Cesium
Cesium is a pale gold element, and it melts at body temperature. Its melting point is 28.5 °C ad boiling point is 671 °C.
Gallium
Gallium is a soft, silver-colored metal when solid. It turns liquid at room temperature. When liquid, it looks silvery white. Its melting point is 29 °C ad boiling point is 2400 °C.
Rubidium
Rubidium is a soft silvery element. It belongs to the alkali metal group. Its melting point is 39 °C ad boiling point is 696 °C.
What are the Elements that exist as Gases at Room Temperature?
Elements that stays Gas at Room Temperature:
- Helium
- Neon
- Argon
- Krypton
- Xenon
- Radon
Molecules that stays Gas at Room Temperature:
- Hydrogen
- Nitrogen
- Oxygen
- Ozone
- Fluorine
- Chlorine
Compounds that stays Gas at Room Temperature:
- Hydrogen fluoride
- Hydrogen chloride
- Hydrogen bromide
- Hydrogen iodide
- Hydrogen cyanide
- Hydrogen sulfide
- Ammonia
- Phosphide
- Methane
- Ethylene
- Acetylene
- Propane
- Butane
- Carbon monoxide
- Carbon dioxide
- Nitric oxide
- Nitrous oxide
- Nitrogen dioxide
- Sulfur dioxide
Conclusion
All of the matter around us is any of these three forms: Solid, Liquid, and Gas. One of the most common substances demonstrating the interrelation between them is water.
Remember you use ice, water, and steam. These three demonstrate three forms of matter.







